Rapid determination of entropy and free energy of mixtures from molecular dynamics simulations with the two-phase thermodynamic model†
Abstract
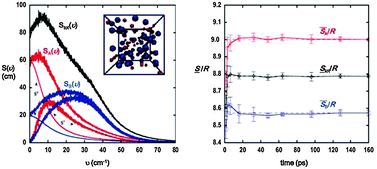
The two-phase thermodynamic (2PT) model is generalized to determine the thermodynamic properties of mixtures. In this method, the vibrational density of states (DoS), obtained from the Fourier transform of the velocity autocorrelation function, and quantum statistics are combined to determine the entropy and free energy from the trajectory of a molecular dynamics simulation. In particular, the calculated DoS is decomposed into a solid-like and a gas-like component through the fluidicity parameter, allowing for treatments for the anharmonic effects in fluids. The 2PT method has been shown to provide reliable thermodynamic properties of

 Please wait while we load your content...
Please wait while we load your content...