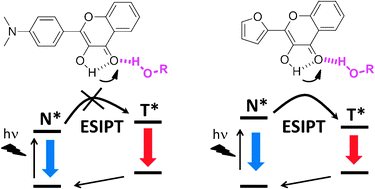
The electronic transitions occurring in 4-(N,N-dimethylamino)-3-hydroxyflavone (DMAF) and 2-furanyl-3-hydroxychromone (FHC) were investigated using the TDDFT method in aprotic and protic solvents. The solvent effect was incorporated into the calculations via the PCM formalism. The H-bonding between solute and protic solvent was taken into account by considering a molecular complex between these molecules. To examine the effect of the H-bond on the ESIPT reaction, the absorption and emission wavelengths as well as the energies of the different states that intervene during these electronic transitions were calculated in acetonitrile, ethanol and methanol. The calculated positions of the absorption and emission wavelengths in various solvents were in excellent agreement with the experimental spectra, validating our approach. We found that in DMAF, the hydrogen bonding with protic solvents makes the ESIPT reaction energetically unfavourable, which explains the absence of the ESIPT tautomer emission in protic solvents. In contrast, the excited tautomer state of FHC remains energetically favourable in both aprotic and protic solvents. Comparing our calculations with the previously reported time-resolved fluorescence data, the ESIPT reaction of DMAF in aprotic solvents is reversible because the emitting states are energetically close, whereas in FHC, ESIPT is irreversible because the tautomer state is below the corresponding normal state. Therefore, the ESIPT reaction in DMAF is controlled by the relative energies of the excited states (thermodynamic control), while in FHC the ESIPT is controlled probably by the energetic barrier (kinetic control).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...