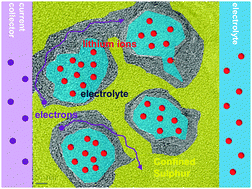
A microporous–mesoporous carbon with graphitic structure was developed as a matrix for the sulfur cathode of a Li–S cell using a mixed carbonate electrolyte. Sulfur was selectively introduced into the carbon micropores by a melt adsorption–solvent extraction strategy. The micropores act as solvent-restricted reactors for sulfur lithiation that promise long cycle stability. The mesopores remain unfilled and provide an ion migration pathway, while the graphitic structure contributes significantly to low-resistance electron transfer. The selective distribution of sulfur in micropores was characterized by X-ray photoelectron spectroscopy (XPS), nitrogen cryosorption analysis, transmission electron microscopy (TEM), X-ray powder diffraction and Raman spectroscopy. The high-rate stable lithiation–delithiation of the carbon–sulfur cathode was evaluated using galvanostatic charge–discharge tests, cyclic voltammetry and electrochemical impedance spectroscopy. The cathode is able to operate reversibly over 800 cycles with a 1.8 C discharge–recharge rate. This integration of a micropore reactor, a mesopore ion reservoir, and a graphitic electron conductor represents a generalized strategy to be adopted in research on advanced sulfur cathodes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...