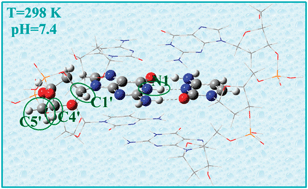
Different deprotonation paths of the radical cation formed by one-electron oxidation of 2′-deoxyguanosine (2dG) sites in DNA have been studied using Density Functional Theory (M05-2X/6-31+G(d,p)) and ONIOM methodology (M05-2X/6-31+G(d,p):PM6) in conjunction with the SMD model to include the solvent effects. Models of increased complexity have been used ranging from the isolated nucleoside to a three unit double-stranded oligomer including the sugar units, the base pairing with cytidine, and the phosphate linkage. The reported results correspond to aqueous solution, at room temperature, and pH = 7.4. Under such conditions it was found that the proton transfer (PT) within the base pair is a minor path compared to the PT between the base pair and the surrounding water. It was also found that the deprotonation of ground-state 2dG˙+ sites mainly yields C centered radicals in the sugar unit, with the largest populations corresponding to C4′˙ and C5′˙, followed by C3′˙. The different aspects of the presented theoretical study have been validated with experimental results.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...