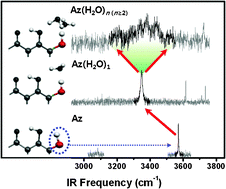
We investigated the hydrogen bonding structures and proton transfer for the hydration complexes of alizarin (Az) produced in a supersonic jet using fluorescence excitation (FE), dispersed laser induced fluorescence (LIF), visible–visible hole burning (HB), and fluorescence detected infrared (FDIR) spectroscopy. The FDIR spectrum of bare Az with two O–H groups exhibits two vibrational bands at 3092 and 3579 cm−1, which, respectively, correspond to the stretching vibration of O1–H1 that forms a strong intramolecular hydrogen bond with the C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O9 carbonyl group and the stretching vibration of O2–H2 that is weakly hydrogen-bonded to O1–H1. For the 1 ∶ 1 hydration complex Az(H2O)1, we identified three conformers. In the most stable conformer, the water molecule forms hydrogen bonds with the O1–H1 and O2–H2 groups of Az as a proton donor and proton acceptor, respectively. In the other conformers, the water binds to the C10
O9 carbonyl group and the stretching vibration of O2–H2 that is weakly hydrogen-bonded to O1–H1. For the 1 ∶ 1 hydration complex Az(H2O)1, we identified three conformers. In the most stable conformer, the water molecule forms hydrogen bonds with the O1–H1 and O2–H2 groups of Az as a proton donor and proton acceptor, respectively. In the other conformers, the water binds to the C10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O10 group in two nearly isoenergetic configurations. In contrast to the sharp vibronic peaks in the FE spectra of Az and Az(H2O)1, only broad, structureless absorption was observed for Az(H2O)n (n ≥ 2), indicating a facile decay process, possibly due to proton transfer in the electronic excited state. The FDIR spectrum with the wavelength of the probe laser fixed at the broad band exhibited a broad vibrational band near the O2–H2 stretching vibration frequency of the most stable conformer of Az(H2O)1. With the help of theoretical calculations, we suggest that the broad vibrational band may represent the occurrence of proton transfer by tunnelling in the electronic ground state of Az(H2O)n (n ≥ 2) upon excitation of the O2–H2 vibration.
O10 group in two nearly isoenergetic configurations. In contrast to the sharp vibronic peaks in the FE spectra of Az and Az(H2O)1, only broad, structureless absorption was observed for Az(H2O)n (n ≥ 2), indicating a facile decay process, possibly due to proton transfer in the electronic excited state. The FDIR spectrum with the wavelength of the probe laser fixed at the broad band exhibited a broad vibrational band near the O2–H2 stretching vibration frequency of the most stable conformer of Az(H2O)1. With the help of theoretical calculations, we suggest that the broad vibrational band may represent the occurrence of proton transfer by tunnelling in the electronic ground state of Az(H2O)n (n ≥ 2) upon excitation of the O2–H2 vibration.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O9 carbonyl group and the stretching vibration of O2–H2 that is weakly hydrogen-bonded to O1–H1. For the 1 ∶ 1 hydration complex Az(H2O)1, we identified three conformers. In the most stable conformer, the
O9 carbonyl group and the stretching vibration of O2–H2 that is weakly hydrogen-bonded to O1–H1. For the 1 ∶ 1 hydration complex Az(H2O)1, we identified three conformers. In the most stable conformer, the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O10 group in two nearly isoenergetic configurations. In contrast to the sharp vibronic peaks in the FE spectra of Az and Az(H2O)1, only broad, structureless absorption was observed for Az(H2O)n (n ≥ 2), indicating a facile decay process, possibly due to
O10 group in two nearly isoenergetic configurations. In contrast to the sharp vibronic peaks in the FE spectra of Az and Az(H2O)1, only broad, structureless absorption was observed for Az(H2O)n (n ≥ 2), indicating a facile decay process, possibly due to 

 Please wait while we load your content...
Please wait while we load your content...