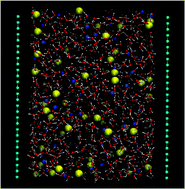
The underlying mechanisms of specific ion effects on structure and dynamics of aqueous solutions have been long debated. On the other hand, the role of polarization at hydrophobic interfaces when aqueous electrolytes are present is of great importance, as it has been observed at the air–vapor interface. In this work, we have explored influence of ionic species on microscopical properties of aqueous sodium halide solutions constrained inside a double layer graphene channel, as a model for a realistic hydrophobic interface. Our systems have been simulated by molecular dynamics techniques, explicitly including polarization in water molecules and ions. Water and ionic density profiles showed the tendency of ionic species to occupy the whole space available, in good agreement with spectroscopic experimental data. The exception to this general behavior was fluoride, which preferred to stay away from interfaces. Two main regions were defined: interfaces and the central part of the slab, the bulklike region. Ionic hydration numbers at interfaces were lower than those at the bulklike area by about one to two units. We have also analyzed water–ion orientations and polarization distributions and obtained a marked dependence on ionic concentration. Residence time of anions suffered important fluctuations and tended to be largest at interfaces. Large variations of the static permittivity between interfacial and bulklike regions were observed. Ionic diffusion was found to be between 10−5 and 10−6 cm2 s−1 and showed to be mainly dependent on the concentration, whereas the type of anion considered and the polarizability had significantly less relevance. Conductivities were found to be dependent on ionic concentrations and the polarizabilities of anions, as well as on the spatial direction considered.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...