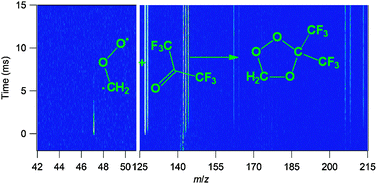
Criegee biradicals, i.e., carbonyl oxides, are critical intermediates in ozonolysis and have been implicated in autoignition chemistry and other hydrocarbon oxidation systems, but until recently the direct measurement of their gas-phase kinetics has not been feasible. Indirect determinations of Criegee intermediate kinetics often rely on the introduction of a scavenger molecule into an ozonolysis system and analysis of the effects of the scavenger on yields of products associated with Criegee intermediate reactions. Carbonyl species, in particular hexafluoroacetone (CF3COCF3), have often been used as scavengers. In this work, the reactions of the simplest Criegee intermediate, CH2OO (formaldehyde oxide), with three carbonyl species have been measured by laser photolysis/tunable synchrotron photoionization mass spectrometry. Diiodomethane photolysis produces CH2I radicals, which react with O2 to yield CH2OO + I. The formaldehyde oxide is reacted with a large excess of a carbonyl reactant and both the disappearance of CH2OO and the formation of reaction products are monitored. The rate coefficient for CH2OO + hexafluoroacetone is k1 = (3.0 ± 0.3) × 10−11 cm3 molecule−1 s−1, supporting the use of hexafluoroacetone as a Criegee-intermediate scavenger. The reactions with acetaldehyde, k2 = (9.5 ± 0.7) × 10−13 cm3 molecule−1 s−1, and with acetone, k3 = (2.3 ± 0.3) × 10−13 cm3 molecule−1 s−1, are substantially slower. Secondary ozonides and products of ozonide isomerization are observed from the reactions of CH2OO with acetone and hexafluoroacetone. Their photoionization spectra are interpreted with the aid of quantum-chemical and Franck–Condon-factor calculations. No secondary ozonide was observable in the reaction of CH2OO with acetaldehyde, but acetic acid was identified as a product under the conditions used (4 Torr and 293 K).

 Please wait while we load your content...
Please wait while we load your content...