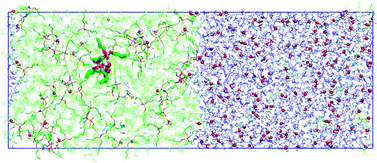
We have studied the extraction of four HA acids (HNO3, HReO4, HClO4, HCl) to a hydrophobic ionic liquid (IL) 1-butyl-3-methylimidazolium-bis(trifluoromethanesulfonyl)amide (BMI+ Tf2N−) at room temperature, in a wide range of acidic concentrations in water. The effect of tributylphosphate (TBP) as co-solvent is investigated. According to experimental observations, water dragging to the IL phase increases with added TBP and/or acids. Acid extraction is found to be weak, however, for the four acids except for concentrated HNO3 (>3 M). Molecular dynamics simulations on model biphasic systems show that TBP is not surface active, but well dissolved in the IL. They also reveal the importance of HA acid model (either totally or half dissociated) and of the TBP content on acid extraction to the IL. Furthermore, they show that “the proton” can be extracted by TBP (H3O+(TBP)3 “complex”) without its A− conjugated base, via a cation transfer mechanism (BMI+ transfer to water). Experiments and simulations show that TBP plays an important role in the mutual solubility between water and ionic liquid, by different amounts, depending on the HA acid. On the other hand, both approaches indicate that a HTf2N containing aqueous solution completely mixes with the [BMI][Tf2N] IL that contains the same Tf2N− anion.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...