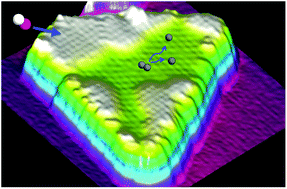
Cobalt is an active metal for a variety of commercially and environmentally significant heterogeneously catalysed processes. Despite its importance, Co's surface chemistry is less studied compared to other key industrial catalyst metals. This stems in part from the difficulties associated with single crystal preparation and stability. Recent advances in scanning probe microscopy have enabled the atomic scale study of the structural, electronic, and magnetic properties of well-defined Co nanoparticles on metal substrates. Such systems offer an excellent platform to investigate the adsorption, diffusion, dissociation, and reaction of catalytically relevant molecules. Here we discuss the current understanding of metal-supported Co nanoparticles, review the limited literature on molecular adsorption, and suggest ways that they can be used to explore Co's rich surface chemistry. Our discussion is accompanied by new high resolution scanning tunnelling microscopy data from our group, which illustrate some of the interesting properties of these complex systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...