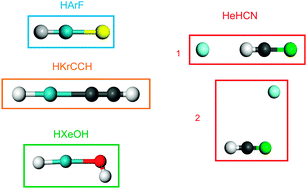
The validity of the description of the DFT approximations currently implemented in plane wave DFT codes (LDA, GGA, meta-GGA, hybrid, GGA + empirical dispersion correction) for interactions between rare gases and open-shell atoms which form materials is poorly known. We have performed a first assessment of the accuracy of these functionals for the description of the bonds formed by helium, argon, krypton and xenon with various open-shell atoms. This evaluation has been done on model molecular systems for which precise experimental data are available and reference post-Hartree–Fock calculations (CCSD(T) using large basis sets) are feasible. The results show that when the rare gas atom shares density with the neighbouring atoms, the GGA functionals yield good geometries and qualitatively correct binding energies, even if these are quite significantly overestimated. The use of hybrid functionals enables us to obtain good geometries and satisfactory binding energies. For compounds in which the rare gas atom forms weak dispersive-like bonding, the accuracy yielded by the various functionals is not as good. No functional gives satisfactory binding energies for all the compounds investigated. Several GGA and hybrid functionals yield correct geometries, even if some isomers are not obtained. One GGA functional (PBE) yields qualitatively correct results for the compounds of the three rare gases and several hybrid functionals give satisfactory energies for He compounds. The addition of an empirical dispersive correction improves the results on association compounds, but several isomers are not found.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...