Remote halogen switch of amine hydrophilicity†
Abstract
Bromide and iodide anions switch hydrogen-bonding patterns in otherwise isostructural dimethanol solvates N-methyl-1,4-diazabicyclo[2.2.2]octanium bromide (dabcoCH3Br·2CH3OH) and analogous iodide (dabcoCH3I·2CH3OH), both synthesized in the high-pressure version of the Menshutkin reaction at 1.2 and 2.4 GPa, respectively. The magnitudes of the high pressure triggering these reactions correspond to identical molecular volumes of both solvates.
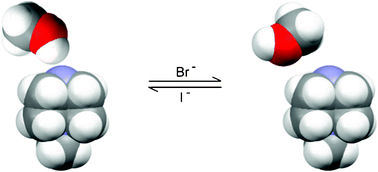

 Please wait while we load your content...
Please wait while we load your content...