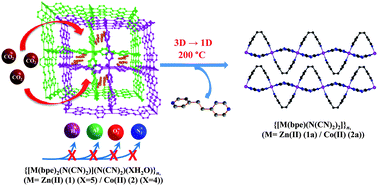
α-Po type 3D porous frameworks, {[M(bpe)2(N(CN)2)]N(CN)2·xH2O}n, (M = Zn(II) (x = 5) (1)/Co(II) (x = 4) (2)) (bpe = 1,2-bis(4-pyridyl)ethane, N(CN)2− = dicyanamide anion) composed of mixed ligand systems have been synthesized and structurally characterized. Upon two-fold interpenetration both 3D frameworks 1 and 2 show a bimodal channel structure; the small channels contain non-coordinated N(CN)2− anions and the bigger channels are occupied by guest water molecules. High framework stability for both compounds was realized by similarity in the PXRD pattern in dehydrated state and even a reversible single-crystal-to-single-crystal transformation for framework 1. Both the frameworks display unprecedented structural transformation from 3D framework to 1D {[M(bpe)(N(CN)2)2]}n (M = Zn(II) (1b)/Co(II) (2b)) coordination chain upon removal of one molecule of bpe and concomitant bridging of non-coordinated N(CN)2− and conformational change (anti to gauche) by another bridging bpe linker. Moreover, the dehydrated solid {[Co(bpe)2(N(CN)2)]N(CN)2}n (2a) exhibits highly selective CO2 uptake relative to a number of adsorbates (H2, N2, O2 and Ar) at 195 K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...