Lewis acid-catalyzed Friedel–Crafts alkylations of 3-hydroxy-2-oxindole: an efficient approach to the core structure of azonazine†
Abstract
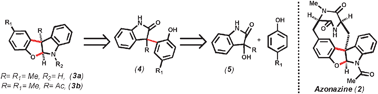
A Lewis acid catalyzed Friedel–Crafts reaction of electron rich aromatics with 3-alkyl-3-hydroxy-2-oxindole (5) has been developed. The methodology provides a straightforward access to the core of

 Please wait while we load your content...
Please wait while we load your content...