Stereoselective hydrofluorination of ynamides: a straightforward synthesis of novel α-fluoroenamides†
Abstract
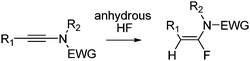
α-Fluoroenamides, potent rigid fluorinated bioisosters of
* Corresponding authors
a
Superacid group in “Glycochimie, Superacide et Chimie des systèmes” team - Université de Poitiers, CNRS UMR 7285 IC2MP, 4, avenue Michel Brunet, F-86022 Poitiers Cedex, France
E-mail:
sebastien.thibaudeau@univ-poitiers.fr
Fax: +33-549453501
Tel: +33-549454588
b Institut Lavoisier de Versailles, UMR CNRS 8180, Université de Versailles St-Quentin, 45 avenue des Etats Unis, 78035 Versailles Cedex, France
c Laboratoire de Chimie Organique, Service de Chimie et PhysicoChimie Organiques, Université Libre de Bruxelles, Avenue F. D. Roosevelt 50, 1050 Brussels, Belgium
α-Fluoroenamides, potent rigid fluorinated bioisosters of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
G. Compain, K. Jouvin, A. Martin-Mingot, G. Evano, J. Marrot and S. Thibaudeau, Chem. Commun., 2012, 48, 5196 DOI: 10.1039/C2CC31768K
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
