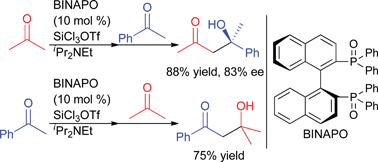
Trichlorosilyl triflate-mediated enantioselective directed cross-aldol reaction between ketones using a chiral phosphine oxide as an organocatalyst†‡
Abstract
Trichlorosilyl triflate-promoted directed cross-
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...