Azaphthalocyanines with fused triazolo rings: formation of sterically stressed constitutional isomers†‡
Abstract
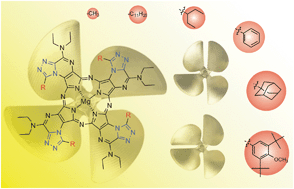
The presented work deals with synthesis and isolation of constitutional isomers of triazolo-fused azaphthalocyanines. Distribution of the isomers did not follow the statistical calculations due to steric effects of the substituents preferring the least sterically stressed C4h isomer.
- This article is part of the themed collection: Porphyrins & Phthalocyanines

 Please wait while we load your content...
Please wait while we load your content...