Enantioselective synthesis of optically active cis-β-thio-α-amino acid derivatives through an organocatalytic cascade thio-Michael/ring opening process†‡
Abstract
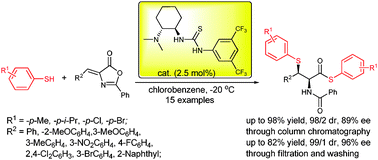
Non-naturally enantioenriched cis-β-thio-α-amino acid derivatives were synthesized through one pot, cascade thio-Michael/ring opening reaction of aromatic
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...