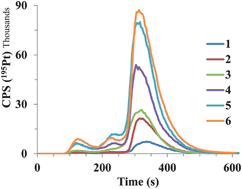
Among platinum (Pt) containing chemotherapeutics, cisplatin (CDDP) is the most widely used. Its distribution in spiked human serum and serum of cancer patients was studied by liquid chromatography with UV and ICP-MS detection. Rapid Pt fractionation was performed by SEC on a HiTrap desalting column. The CIM DEAE-1 fast monolithic column was used in speciation analysis of CDDP for the first time. Complementary to monolithic chromatography, Pt speciation was also performed by FPLC on a strong anion-exchange Mono Q particle packed column. To study CDDP interactions with proteins, synthetic solutions of single standard proteins (albumin (HSA), transferrin (Tf) and γ-globulins (IgG)) or their mixtures, and human serum samples were spiked with CDDP and incubated at 37 °C for 24 h. Results of fractionation showed that more than 80% of Pt in serum was eluted with proteins and the remaining portion as low molecular mass species. Serum proteins were efficiently separated by anion-exchange chromatography on FPLC Mono Q or CIM DEAE-1 columns. Both complementary speciation procedures gave statistically comparable results. In spiked serum samples about 83 to 87% of Pt was eluted with HSA, 3 to 4.5% with Tf and the remaining 8.5 to 14%, which represents unbound CDDP, with a solvent front. The developed CIM-DEAE-1 fast monolithic chromatography procedure was applied to Pt speciation in serum of cancer patients receiving CDDP-based chemotherapy. In these samples Pt was found to be bound as follows: 87 to 93% to HSA, 2.6 to 6.4% to Tf, and 4.2 to 6.6% as unbound CDDP species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...