Study on electrochemical oxidation behaviors and the diffusion mechanism of hydroquinone at pre-anodized carbon paste electrode by cyclic voltammetry†
Abstract
A functional pre-anodized carbon paste
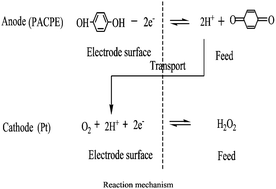
- This article is part of the themed collection: Future Electroanalytical Developments

 Please wait while we load your content...
Please wait while we load your content...