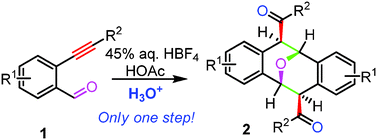
Brønsted acid-promoted dimerization of o-alkynylbenzaldehydes: a one-step synthesis of functionalized Kagan's ether analogues†‡
Abstract
The Brønsted acid-promoted dimerization of o-alkynylbenzaldehydes has been discovered and studied in the presence of 45% aq. HBF4 in

 Please wait while we load your content...
Please wait while we load your content...