Exploring the topography of free energy surfaces and kinetics of cytochrome c oxidases interacting with small ligands†
Abstract
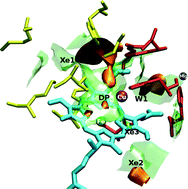
Free energy landscape explorations have been performed for Cytochrome c Oxidases, aa3 from Paracoccus denitrificans and ba3 from Thermus thermophilus, interacting with small gas molecules (CO, NO, O2), as well as Xe. The calculations were carried out with thermodynamic perturbation theory, the validity of which has been examined by previous molecular dynamics calculations. This approach allows us to bypass the immense computational time required in such problems. The free energy surfaces are constructed as functions of the three Cartesian coordinates of the center of mass of the

 Please wait while we load your content...
Please wait while we load your content...