Organocatalytic asymmetric Michael reaction with acylsilane donors†
Abstract
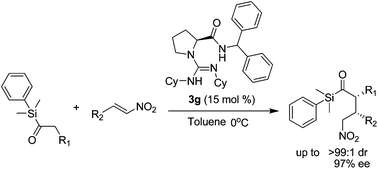
We have developed an organocatalytic asymmetric Michael reaction of acylsilane through the selection of acylsilane substrates and organocatalysts, thus creating a rare example of acylsilane α-alkylation with a chiral

 Please wait while we load your content...
Please wait while we load your content...