Diastereoselective 1,3-dipolar cycloaddition of pyrylium ylides with chiral enamides†
Abstract
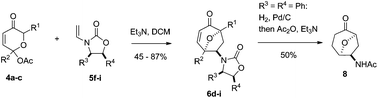
Chiral enamides 5f–i were found to react with pyrylium ylides to give cycloadducts 6d–i in good yields with an excellent level of stereoselectivity. The chiral auxiliary was successfully removed on hydrogenolysis of compound 6f in continuous flow (H-Cube) resulting in the first asymmetric synthesis of complex amine 8.
 Please wait while we load your content...
Please wait while we load your content...