Understanding structural defects in lithium-rich layered oxide cathodes
Abstract
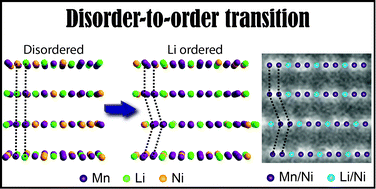
Planar defects in lithium-rich layered ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m state to a lithium-ordered C2/m state. This disorder-to-order transition resulted in three orientation variants, namely [100], [110], and [1
m state to a lithium-ordered C2/m state. This disorder-to-order transition resulted in three orientation variants, namely [100], [110], and [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]. The fundamental mechanism behind the observed defects is a shear of ±b/3[010] on the (001) transition metal planes, which is equivalent to the point group operations lost during the disorder-to-order transition. These displacements also produced twins and single unit cells with P3112 symmetry. Lithium-rich layered
0]. The fundamental mechanism behind the observed defects is a shear of ±b/3[010] on the (001) transition metal planes, which is equivalent to the point group operations lost during the disorder-to-order transition. These displacements also produced twins and single unit cells with P3112 symmetry. Lithium-rich layered

 Please wait while we load your content...
Please wait while we load your content...