The first example of the direct asymmetric conjugate addition of aldehydes to a methylenemalonate promoted by an axially chiral amino diol catalyst†
Abstract
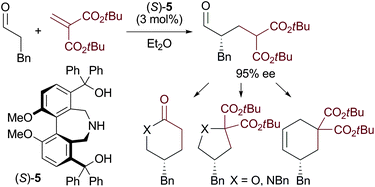
A methylenemalonate could be employed as a reactive equivalent of a three carbon Michael acceptor such as acrylate in a direct asymmetric

 Please wait while we load your content...
Please wait while we load your content...