Synthesis of flinderoles B and C by a gold-catalyzed allene hydroarylation†
Abstract
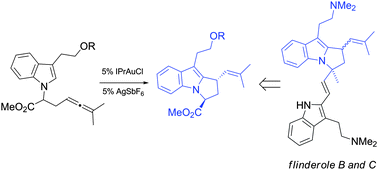
The recent development of new gold(I) catalysis methodologies has opened the door to new disconnections for the total synthesis of bioactive complex molecules. Below is described the application of a gold(I)-catalyzed hydroarylation of an

 Please wait while we load your content...
Please wait while we load your content...