Thermolysis of the structurally characterized hafnium phosphide complex, CpCp*HfMe(PHPh) (2), resulted in formation of the triphosphanato compound CpCp*Hf(P3Ph3) (3). Trapping reactions with PMe3 gave evidence for an intermediate phosphinidene complex, which was corroborated by synthesis of the related 2,6-dimesitylphenyl derivative CpCp*(Me3P)Hf![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(dmp) (8). The hafnocene phenylphosphinidene intermediate can also be intercepted by a [2 + 2]-cycloaddition reaction with 2-butyne. However, reaction of 2 with either xylyl isocyanide or benzophenone gives insertion into the hafnium methyl bond. Under thermolytic conditions, metal dichloro complexes can efficiently intercept a phosphinidene fragment from 2 in a unique phosphinidene ligand exchange reaction. Thus, complex 2 reacts with (dippe)PtCl2 (11, dippe = 1,2-bis(diisopropylphosphino)ethane) and [N(Np)Ar]3TaCl2 (14, Np = neopentyl, Ar = 3,5-Me2C6H3) to afford phosphinidene complexes [(dippe)Pt(μ-PPh)]2 (12) and [N(Np)Ar]3Ta
P(dmp) (8). The hafnocene phenylphosphinidene intermediate can also be intercepted by a [2 + 2]-cycloaddition reaction with 2-butyne. However, reaction of 2 with either xylyl isocyanide or benzophenone gives insertion into the hafnium methyl bond. Under thermolytic conditions, metal dichloro complexes can efficiently intercept a phosphinidene fragment from 2 in a unique phosphinidene ligand exchange reaction. Thus, complex 2 reacts with (dippe)PtCl2 (11, dippe = 1,2-bis(diisopropylphosphino)ethane) and [N(Np)Ar]3TaCl2 (14, Np = neopentyl, Ar = 3,5-Me2C6H3) to afford phosphinidene complexes [(dippe)Pt(μ-PPh)]2 (12) and [N(Np)Ar]3Ta![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh (15), respectively, in good isolated yields. A derivative of 12, [(dippe)Pt]2(μ-PPh) (13), was structurally characterized.
PPh (15), respectively, in good isolated yields. A derivative of 12, [(dippe)Pt]2(μ-PPh) (13), was structurally characterized.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P(dmp) (8). The hafnocene phenylphosphinidene intermediate can also be intercepted by a [2 + 2]-cycloaddition reaction with
P(dmp) (8). The hafnocene phenylphosphinidene intermediate can also be intercepted by a [2 + 2]-cycloaddition reaction with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh (15), respectively, in good isolated yields. A derivative of 12, [(dippe)Pt]2(μ-PPh) (13), was structurally characterized.
PPh (15), respectively, in good isolated yields. A derivative of 12, [(dippe)Pt]2(μ-PPh) (13), was structurally characterized.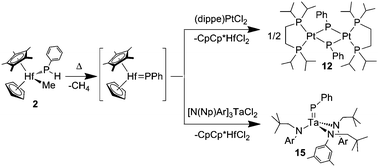

 Please wait while we load your content...
Please wait while we load your content...