Stereoselective interaction of ketoprofen enantiomers with β-cyclodextrin: ground state binding and photochemistry
Abstract
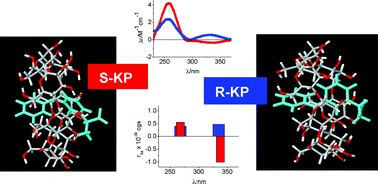
The chiral recognition ability of β-cyclodextrin (β-CyD) vs.S- and R-ketoprofen (KP) enantiomers has been studied by circular dichroism (CD),

 Please wait while we load your content...
Please wait while we load your content...