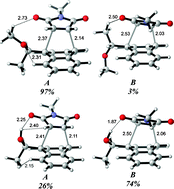
In this study, the origins of diastereoselectivity in the hydrogen bonding assisted Diels–Alder reactions of chiral dienes with achiral dienophiles have been investigated with density functional methods. The distortion/interaction model has been applied to shed light on the origins of selectivity. C9-Substituted chiral anthracene templates (R = (CH3)(OCH3)(H), R = (CH3)(OH)(H), R = (CH3)(CH2CH3)(H) and R = (-CH2-C(CH3)(OCH3)(H)) are used to rationalize the role of a stereogenic center and H-bonding on the product distribution ratio. Even though hydrogen bonding increases the reactivity of the diene, the stereoselectivity is reduced because of the hydrogen bonding capacity of both diastereomeric transition states. The interaction energies of the studied anthracene templates with N-methyl maleimide at the transition state correlate linearly with an increase in reactivity. The selectivity is determined by both favorable distortion and interaction energies. The π-facial selectivity induced by the presence of a chiral auxiliary in 1-substituted 1,3-pentadienes (R1 = (CH3)(OCH3)(H) and R1 = (CH3)(OH)(H)) has also been modeled in order to rationalize the role of the stereogenic center and H-bonding on the stereoselectivity of an aliphatic diene. In both parts, the product distribution ratios calculated from Boltzmann distributions based on Gibbs free energies are in reasonable agreement with the experimental results. Finally the role of OH-substituted five-membered pyrrolidine on C9 of anthracene is investigated since the successful usage of the conformationally rigid pyrrolidines in asymmetric synthesis is well known. Overall, both in the acyclic system and in anthracene, the facilitation due to H-bonding is reflected in the interaction energies: the higher the difference in interaction energies in the transition structures of the two diastereomers, the more selective the H-bonding assisted Diels–Alder reaction is.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...