6,6-Spiroimine analogs of (−)-gymnodimine A: synthesis and biological evaluation on nicotinic acetylcholine receptors†
Abstract
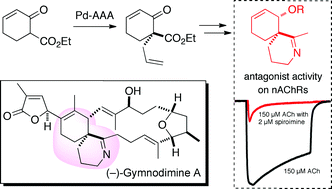
Simple models of the spiroimine core of (−)-gymnodimine A have been synthesized in racemic and optically active forms. The quaternary carbon of the racemic spiroimines was created by

 Please wait while we load your content...
Please wait while we load your content...