Synthesis of (1R,2R)-DPEN-derived triazolium salts and their application in asymmetric intramolecular Stetter reactions†
Abstract
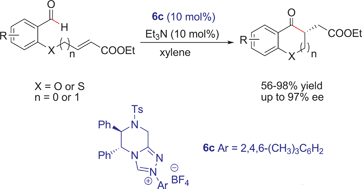
A series of novel chiral triazolium salts has been synthesized from readily available (1R,2R)-DPEN and found to be efficient for the enantioselective intramolecular Stetter reaction. With 10 mol% of the

 Please wait while we load your content...
Please wait while we load your content...