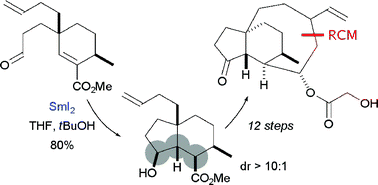
A stereoselective, Sm(ii)-mediated approach to decorated cis-hydrindanes: synthetic studies on faurinone and pleuromutilin†‡
Abstract
The cis-hydrindane motif is found in a number of natural products that display important biological activity. A flexible, stereoselective approach to the framework has been developed that features highly diastereoselective, SmI2-mediated cyclisations. The strategy has been exploited in the first synthesis of the proposed structure of faurinone and an approach to the skeleton of the antibacterial natural product,
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...