An amyloid-like fibril-forming supramolecular cross-β-structure of a model peptide: a crystallographic insight†
Abstract
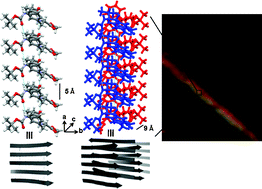
The peptideBoc-Val-Phe-OMe 1 bearing sequence similarity with the central hydrophobic cluster (CHC) of Alzheimer's Aβ18-19peptide self-assembles to produce amyloid-like straight unbranched fibrils as examined by

 Please wait while we load your content...
Please wait while we load your content...