A general approach to N-heterocyclic scaffolds using domino Heck–aza-Michael reactions†
Abstract
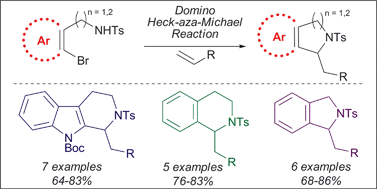
Palladium-catalyzed domino Heck–aza-Michael reactions for the synthesis of a series of C1-substituted tetrahydro-β-carbolines, tetrahydroisoquinolines and isoindolines are described. The domino process involves the initial intermolecular
 Please wait while we load your content...
Please wait while we load your content...