Computational and experimental investigations of mono-septanoside binding by Concanavalin A: correlation of ligand stereochemistry to enthalpies of binding†
Abstract
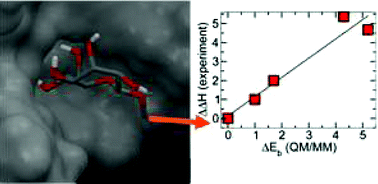
Structure-energy relationships for a small group of pyranose and septanose mono-saccharide

 Please wait while we load your content...
Please wait while we load your content...