Octahedral niobium cluster-based solid state halides and oxyhalides: effects of the cluster condensation via an oxygen ligand on electronic and magnetic properties†
Abstract
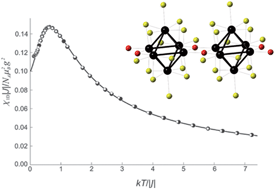
The influences of an oxygen
- This article is part of the themed collection: In honour of Didier Astruc

 Please wait while we load your content...
Please wait while we load your content...