Electrochromic devices based on in situ polymerised EDOT and Prussian Blue: influence of transparent conducting oxide and electrolyte composition—towards up-scaling†‡
Abstract
Inorganic/organic (hybrid) complementary electrochromic devices (ECDs) of the type [transparent conducting
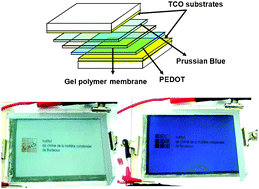
- This article is part of the themed collection: In honour of Didier Astruc

 Please wait while we load your content...
Please wait while we load your content...