Novel benzyl-substituted N-heterocyclic carbene–silver acetate complexes: synthesis, cytotoxicity and antibacterial studies†
Abstract
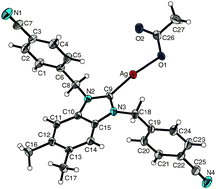
From the reaction of
* Corresponding authors
a
Conway Institute of Biomolecular and Biomedical Research, Centre for Synthesis and Chemical Biology (CSCB), UCD School of Chemistry and Chemical Biology, University College Dublin, Belfield, Dublin 4, Ireland
E-mail:
matthias.tacke@ucd.ie
From the reaction of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
