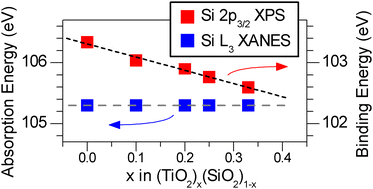
The (TiO2)x(SiO2)1−x system (0 ≤ x ≤ 0.33) was synthesized by the sol–gel method and investigated by X-ray absorption near-edge spectroscopy (XANES) and X-ray photoelectron spectroscopy (XPS). The use of both hard (Ti K-edge) and soft (Ti L-edge) X-rays provides a useful way to monitor changes in the bulk and surface, respectively, of these amorphous materials. The average CN of both bulk-Ti and surface-Ti increases with greater x in the chemical formula, due to the larger ionic radius of Ti. Comparison of Ti K- and L-edge spectra of annealed samples revealed that Ti atoms at the surface have a higher average CN than in the bulk, likely due to the presence of surface hydroxide and water groups that can coordinate to the Ti centres. The O K-edge, Ti L-edge, and Si L-edge XANES absorption energies showed little to no change with Ti content, while the O 1s, Ti 2p, and Si 2p XPS BEs were found to decrease with increasing Ti content due to nearest-neighbour and next-nearest-neighbour effects, which lead to increased final-state relaxation. The degree of final-state relaxation is more significant than previously believed for these amorphous powders.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...