Abstract
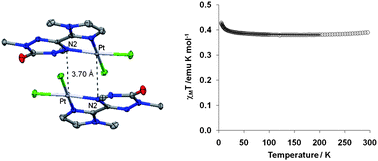
The synthesis, structures, and magnetic properties of several Pd(II) and Pt(II) complexes of verdazyl radicals are presented. Reactions of bidentate verdazyl radicals with (RCN)2MCl2 (R = Me or Ph; M = Pd or Pt) produced square planar (verdazyl)MCl2 complexes, three based on palladium and two on platinum. Solution spectroscopic studies indicate that the verdazyl
- This article is part of the themed collection: Celebrating the 70th birthday of Professor Fred Wudl

 Please wait while we load your content...
Please wait while we load your content...