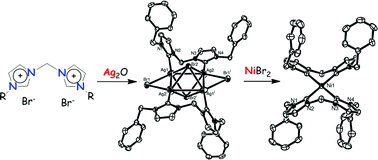
Air and moisture stable homoleptic bis(diimidazolylidine)nickel(II) complexes, ([(diNHC)2Ni]2+) 3a,b and their corresponding silver(I) 4a,b and palladium(II) 5a,b complexes were synthesized and characterized by NMR and single crystal X-ray analysis. The catalytic potential of complex 3a was assessed in Mizoroki–Heck and Suzuki–Miyaura coupling reactions. In the Suzuki–Miyaura coupling reaction, nickel precatalyst 3a was active for the coupling of aryl chlorides as well as aryl fluorides. The analogously synthesized Pd(II) complexes resulted in formation of (diNHC)PdCl2 species which were not active for the coupling of aryl fluorides. For the Mizoroki–Heck reaction, it was found that aryl iodides could be activated in the absence of nickel or palladium precatalysts when using Na2CO3 or NEt3 as base while aryl iodides and aryl bromides could be activated in the Suzuki–Miyaura reaction sans precatalyst when K3PO4 was used as base.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...