Synthesis, structure and magnetic properties of nanocrystalline YMnO3†
Abstract
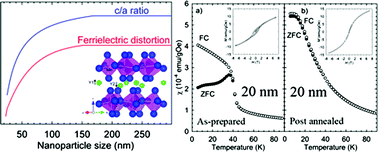
Nanocrystalline YMnO3 has been prepared by wet chemical synthesis routes to obtain crystallites with sizes from 20 nm to bulk material. The crystal structure of hexagonal YMnO3 nanocrystallites smaller than 80 nm deviates from bulk material in terms of unit cell distortion and unit cell volume. The ferrielectric displacements of Y3+

 Please wait while we load your content...
Please wait while we load your content...