The coordination properties of the EN ligands N-(2-pyridinyl)amino-diphenylphosphine sulfide, N-(2-pyridinyl)amino-diisopropylphosphine sulfide, N-(2-pyridinyl)amino-diphenylphosphine selenide, N-(2-pyridinyl)amino-diisopropylphosphine selenide towards copper(I) precursors CuX (X = Br, I), [Cu(IPr)Cl] (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene), and [Cu(CH3CN)4]PF6 were studied. Treatment of CuX with EN ligands resulted in the formation of tricoordinate complexes of the type [Cu(κ2(E,N)-EN)X]. The reaction of [Cu(IPr)Cl] with EN ligands, followed by halide abstraction with AgSbF6, afforded cationic tricoordinate complexes [Cu(κ2(S,N)-EN)(IPr)]+, while the reaction of [Cu(CH3CN)4]+ with two equivalents of EN ligands yielded tetrahedral complexes [Cu(κ2(E,N)-EN)2]+. Halide removal from [Cu(κ2(S,N)-SN)I] with silver salts in the presence of L = CH3CN and CNtBu afforded dinuclear complexes of the type [Cu(κ2(S,N),μ(S)-SN)(L)]22+ containing bridging SN ligands. With the terminal alkynes HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me and HC
CC6H4Me and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4OMe, complexes of the formula [Cu(κ2(S,N)-SN-iPr)(η2-HC
CC6H4OMe, complexes of the formula [Cu(κ2(S,N)-SN-iPr)(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me)]+ and [Cu(κ2(S,N)-SN-iPr)(η2-HC
CC6H4Me)]+ and [Cu(κ2(S,N)-SN-iPr)(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4OMe)]+ were obtained. The mononuclear nature of these compounds was supported by DFT calculations. Most complexes were also characterized by X-ray crystallography.
CC6H4OMe)]+ were obtained. The mononuclear nature of these compounds was supported by DFT calculations. Most complexes were also characterized by X-ray crystallography.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me and HC
CC6H4Me and HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4OMe, complexes of the formula [Cu(κ2(S,N)-SN-iPr)(η2-HC
CC6H4OMe, complexes of the formula [Cu(κ2(S,N)-SN-iPr)(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4Me)]+ and [Cu(κ2(S,N)-SN-iPr)(η2-HC
CC6H4Me)]+ and [Cu(κ2(S,N)-SN-iPr)(η2-HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4OMe)]+ were obtained. The mononuclear nature of these compounds was supported by DFT calculations. Most complexes were also characterized by
CC6H4OMe)]+ were obtained. The mononuclear nature of these compounds was supported by DFT calculations. Most complexes were also characterized by 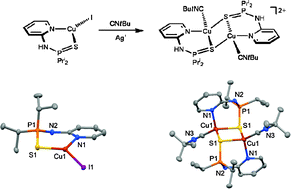

 Please wait while we load your content...
Please wait while we load your content...