Oxidative cyclization of thiosemicarbazone: an optical and turn-on fluorescent chemodosimeter for Cu(ii)†
Abstract
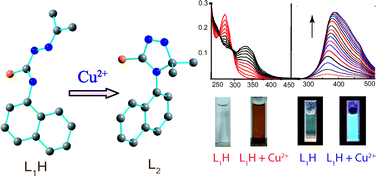
A weakly fluorescent thiosemicabazone (L1H) was found to be a selective optical and “turn-on” fluorescent chemodosimeter for Cu2+ ion in aqueous medium. A significant fluorescence enhancement along with change in color was only observed for Cu2+ ion; among the other tested metal ions (viz.Na+, K+, Mg2+, Ca2+, Cr3+, Zn2+, Cd2+, Hg2+, Pb2+, Ag+, Ni2+, Co2+, Fe3+ and Mn2+). The Cu2+ selectivity resulted from an oxidative cyclization of the weak fluorescent L1H into highly fluorescent rigid

 Please wait while we load your content...
Please wait while we load your content...