On the mechanism of water oxidation by a bimetallic manganese catalyst: A density functional study†
Abstract
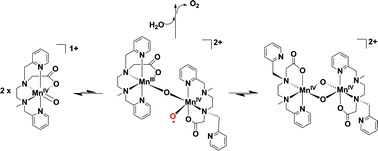
Density functional theory is used to explore possible mechanisms that lead to water
- This article is part of the themed collection: Contributions of inorganic chemistry to energy research

 Please wait while we load your content...
Please wait while we load your content...