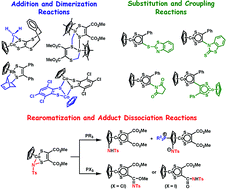
Research progress in the organometallic dithiolene complexes such as [Cp(or Cp*)M(dithiolene)] (M = Co, Rh, Ir, Ni), [(C6R6)Ru(dithiolene)] and [(C4R4)Pt(dithiolene)] complexes during the past decade is described and the reactivities, structures and electrochemical behavior are summarized in this paper. The five-membered metalladithiolene ring (MS2C2) undergoes addition reactions to the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond to form 18-electron adducts by an imido, alkylidene, alkene or norbornene group and also undergoes dimerizations on the basis of the unsaturation in the ring. The aromaticity of the ring causes substitution reactions on the dithiolene carbon by a C-centered radical, S-centered radical or succinimide group when the ring has a C–H bond. Furthermore a dithiolene–dithiolene homo-coupling reaction by an acid or dithiolene–aryl cross-coupling occurs based on the aromaticity in the ring. Dissociations of the 18-electron adducts are observed by those thermolyses, photolyses, electrochemical redox reactions and other chemical reactions with tertiary phosphorus compounds. One representative example of them is the imido adduct dissociation with PR3 under heating toward the intramolecular imido migration to a Cp ligand. Since all products are rearomatized by those adduct dissociations, it is concluded that the ‘coexistence of aromaticity and unsaturation’ in the metallacycle mediates the diverse chemical reactions.
S bond to form 18-electron adducts by an imido, alkylidene, alkene or norbornene group and also undergoes dimerizations on the basis of the unsaturation in the ring. The aromaticity of the ring causes substitution reactions on the dithiolene carbon by a C-centered radical, S-centered radical or succinimide group when the ring has a C–H bond. Furthermore a dithiolene–dithiolene homo-coupling reaction by an acid or dithiolene–aryl cross-coupling occurs based on the aromaticity in the ring. Dissociations of the 18-electron adducts are observed by those thermolyses, photolyses, electrochemical redox reactions and other chemical reactions with tertiary phosphorus compounds. One representative example of them is the imido adduct dissociation with PR3 under heating toward the intramolecular imido migration to a Cp ligand. Since all products are rearomatized by those adduct dissociations, it is concluded that the ‘coexistence of aromaticity and unsaturation’ in the metallacycle mediates the diverse chemical reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond to form 18-electron adducts by an imido, alkylidene,
S bond to form 18-electron adducts by an imido, alkylidene, 

 Please wait while we load your content...
Please wait while we load your content...