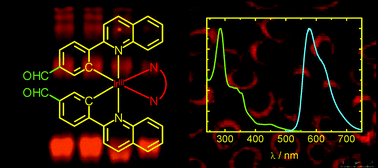
Four new luminescent cyclometallated iridium(III) bis(quinolylbenzaldehyde) diimine complexes [Ir(qba)2(N⁁N)](PF6) (Hqba = 4-(2-quinolyl)benzaldehyde, N⁁N = 2,2′-bipyridine, bpy (1); 1,10-phenanthroline, phen (2); 3,4,7,8-tetramethyl-1,10-phenanthroline, Me4-phen (3); 4,7-diphenyl-1,10-phenanthroline, Ph2-phen (4)) have been synthesised and characterised, and their electronic absorption, emission and electrochemical properties investigated. The X-ray crystal structures of complexes 1 and 2 have been determined. Upon irradiation, complexes 1–4 exhibited intense and long-lived orange-yellow emission in fluid solutions at 298 K and in alcohol glass at 77 K. The emission has been assigned to a triplet intra-ligand (3IL) excited state associated with the qba ligand, probably with mixing of some triplet metal-to-ligand charge-transfer (3MLCT) (dπ(Ir) → π*(qba)) character. Reductive amination reactions of complexes 1–4 with the protein bovine serum albumin (BSA) afforded the bioconjugates 1-BSA–4-BSA, respectively. Upon photoexcitation, these bioconjugates displayed intense and long-lived 3MLCT (dπ(Ir) → π*(N⁁C)) emission in aqueous buffer at 298 K. The cross-linked nature of the Ir–BSA bioconjugates has been verified by SDS-PAGE. Additionally, the cytotoxicity of the complexes towards human cervix epithelioid carcinoma (HeLa) cells has been examined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assays, and the cellular uptake of complex 4 has been investigated by laser-scanning confocal microscopy and flow cytometry.

 Please wait while we load your content...
Please wait while we load your content...