Electronic and steric effects on the photo-induced C → E ring-opening of structurally modified furylfulgides
Abstract
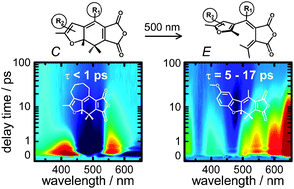
The ultrafast C → E ring-opening reactions of four selectively modified furylfulgides have been studied by means of ultrafast broadband transient absorption

 Please wait while we load your content...
Please wait while we load your content...