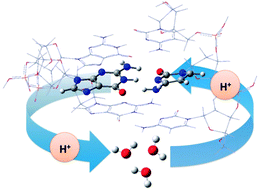
In DNA, base pairs are involved in two reciprocal interactions: interbase hydrogen bonds and stacking. Furthermore, base pairs also undergo the effects of the external entities present in the biological environment, such as water molecules and cations. In this contribution, the double spontaneous mutation has been studied with hybrid theoretical tools in a DNA-embedded guanine–cytosine model accounting for the impact of the first hydration shell. According to our findings, the combination of the neighboring base pairs and surrounding water molecules plays a crucial role in the double proton transfer. Indeed, as a consequence of these interactions, the double proton transfer (DPT) mechanism is altered: on the one hand, stacking and hydration strongly affect the geometry of base pairs, and, on the other hand, vicinal water molecules may play an active role in the tautomeric equilibrium by catalyzing the proton transfer reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...