Electron solvation and solvation-induced crystallization of an ammonia film on Ag(111) studied by 2-photon photoemission†
Abstract
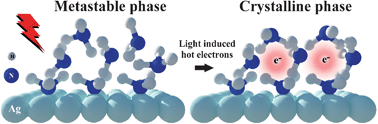
Intermolecular interaction plays a crucial role in electron solvation in the condensed phase. Here, we present a femtosecond time-resolved and angle-resolved 2-photon photoemission (2PPE) study on the dynamics of electron solvation in a 2-dimensional ammonia film on a metal substrate. While the weakly chemisorbed first monolayer (ML) supports delocalized image-potential (

 Please wait while we load your content...
Please wait while we load your content...